lewis structure for na|The Sodium Lewis Dot Diagram: Understanding the Bonding Patterns o : Pilipinas Lewis structures can also be useful in predicting molecular geometry in conjunction with hybrid orbitals. A compound may have multiple resonance forms that . We would like to show you a description here but the site won’t allow us.
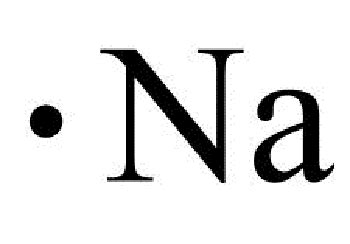
lewis structure for na,A step-by-step explanation of how to draw the Lewis dot structure for Na (Sodium). I show you where Sodium is on the periodic table and how to determine how . A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses .
Learn how to write Lewis symbols and structures for atoms using dots to represent valence electrons. Find out the number of valence electrons for each element from the periodic table and how to achieve a stable octet . Lewis structures can also be useful in predicting molecular geometry in conjunction with hybrid orbitals. A compound may have multiple resonance forms that .The Lewis dot diagram for sodium consists of the symbol “Na” surrounded by a single dot, representing the valence electron. The Lewis dot diagram for sodium can be further . Lewis used simple diagrams (now called Lewis diagrams) to keep track of how many electrons were present in the outermost, or valence, shell of a given atom. .Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that .
A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his 1916 article The Atom and the Molecule.lewis structure for naLearning Objectives. By the end of this section, you will be able to: Write Lewis symbols for neutral atoms and ions. Draw Lewis structures depicting the bonding in simple molecules. Thus far in this chapter, we .
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. . we note that the Na atom has a single valence electron in its Lewis .When the Lewis structure of an ion is written, the entire structure is placed in brackets, and the charge is written as a superscript on the upper right, outside of the brackets. For example, consider the ammonium ion, . For example, in the reaction of Na (sodium) and Cl (chlorine), each Cl atom takes one electron from a Na atom. . The Lewis Structure of this reaction is (here we will consider one chlorine atom, rather than Cl 2) is: The arrow indicates the transfer of the electron from sodium to chlorine to form the Na + metal ion and the Cl-chloride ion.
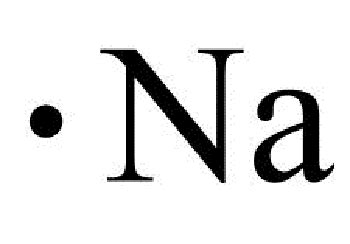
The Lewis Structure for the Salt NaCl depicts two ions with complete octets in their outer shells of electrons. As a result, there is a positive charge on sodium (due to one lost electron) and a negative charge on chlorine (due to one electron gained). . (Na) is the metal that will give away the one electron and forms Na + ion, .The Lewis dot diagram is a visual representation of the valence electrons in an atom. It helps to understand the bonding and structure of molecules. In the case of sodium (Na), the Lewis dot diagram shows the arrangement of its valence electrons. For sodium, the atomic number is 11, and it has an electron configuration of 2-8-1.
Write the Lewis symbol for each atom or ion. b. Na+. Skip to main content. General Chemistry Start typing, then use the up and down arrows to select an option from the list. . Lewis Dot Structure for elements. MooMooMath and Science. 394. views. 1. rank. 04:41. Lewis Dot Structures. Professor Dave Explains. 356. views. 2. rank. 04:03. Lewis .
A compound with a molar mass of about 28 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Write the Lewis structure for a molecule of the compound. A compound with a molar mass of about 42 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Write the Lewis structure for a molecule of the compound.For example, in the reaction of Na (sodium) and Cl (chlorine), each Cl atom takes one electron from a Na atom. . The Lewis Structure of this reaction is (here we will consider one chlorine atom, rather than Cl 2) is: The arrow indicates the transfer of the electron from sodium to chlorine to form the Na + metal ion and the Cl-chloride ion. 10.3: Lewis Structures of Ionic Compounds- Electrons Transferred Expand/collapse global location 10.3: Lewis Structures of Ionic Compounds- Electrons Transferred . It is rather reactive, however, and does not require a lot of energy to remove that electron to make the Na + ion. We could remove another electron by adding even .
Lewis structures (also known as Lewis dot diagrams, electron dot diagrams,"Lewis Dot formula" Lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well .lewis structure for na The Sodium Lewis Dot Diagram: Understanding the Bonding Patterns oThe concept regarding Lewis's structure of any molecule or atom was coined by G.N. Lewis in the year 1916. This concept of representing molecules/atoms is independent of the non-valence electrons. Answer and Explanation: 1. . Draw the Lewis Structure for the following element: Na. . The most stable structure has the least formal charge. In a stable structure, adjacent atoms should have formal charges of opposite signs. The more stable the structure, the more it contributes to the resonance structure of the molecule or ion. All three structures above are the same, only the double bond rotates.The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honour of the American chemist Gilbert Newton Lewis. Lewis dot structures also called electron dot structures are .Na: 1, Mg: 2, B: 3, C: 4, N: 5. Drawing Lewis Structures. In short, these are the steps you need to follow for drawing a Lewis structure: 1. Write the correct skeletal structure for the molecule. * Hydrogen atoms are always terminal (only one bond) * Put more electronegative elements in terminal positions. 2.
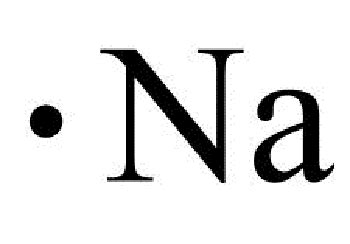
For example, in the reaction of Na (sodium) and Cl (chlorine), each Cl atom takes one electron from a Na atom. . The Lewis Structure of this reaction is (here we will consider one chlorine atom, rather than Cl 2) is: The arrow indicates the transfer of the electron from sodium to chlorine to form the Na + metal ion and the Cl-chloride ion.
Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.10.3: Lewis Structures of Ionic Compounds- Electrons Transferred Expand/collapse global location 10.3: Lewis Structures of Ionic Compounds- Electrons Transferred . It is rather reactive, however, and does not require a lot of energy to remove that electron to make the Na + ion. We could remove another electron by adding even more energy to . A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc.
lewis structure for na|The Sodium Lewis Dot Diagram: Understanding the Bonding Patterns o
PH0 · The Sodium Lewis Dot Diagram: Understanding the Bonding Patterns o
PH1 · The Sodium Lewis Dot Diagram: Understanding the Bonding
PH2 · Lewis Electron Dot Structures
PH3 · Lewis Dot Symbols and Lewis Structures (Writing Lewis Symbols for At
PH4 · Lewis Dot Symbols and Lewis Structures (Writing
PH5 · Lewis Dot Structure for Sodium (Na)
PH6 · How to Draw the Lewis Dot Structure for Na+ (Sodium ion)
PH7 · Drawing Lewis Structures
PH8 · 9.2: Lewis Electron Dot Diagrams
PH9 · 5.3: Lewis Diagrams
PH10 · 4.4 Lewis Symbols and Structures – General
PH11 · 12.1: Lewis Structures